brrly bables about chemistry (atoms and modern atomic theory)
ok so yesterday at the end of my blog post i said i was going to explain more about density and mass and volume but really there's not much more to explain except on how to find volume with density and mass but its pretty simple all you have to do is divide density by mass and boom!!! so yeah i don't really need to explain any more so have fun finding density and mass and volume and stuff like that
ALSO i have a chart for the density of different elements
ok so moving on from density!! inn the textbook its talking about modern atomic theory and atoms and what theyre made up of and why theyre so important in chemistry so i will now be talking about them to actually focus
ALSO i think im gonna try learning astronomy too it seems interesting also my school has access to this website with a bunch of articles and it has a lot of information and i think that's where im going to start my research on things that i want to learn about so i can write about them in my blog posts so YAY WOOHOO MORE INFORMATION!!!
ok so we have more vocab so woohoo
atom - The smallest piece of an
element that maintains the
identity of that element.
modern atomic theory - The concept that atoms play a
fundamental role in chemistry.
below are the three statements of the modern atomic theory:
1. All matter is composed of atoms.
2. Atoms of the same element are the same; atoms of different elements
are different.
3. Atoms combine in whole-number ratios to form compounds.
electron - A tiny subatomic particle with
a negative charge.
proton - A subatomic particle with a
positive charge.
neutron - A subatomic particle with no
charge.
^^they all make up an atom btw!! that's why they're called subatomic particles
here's the chart for the subatomic particles
nuclear model (this word isn't really relevant but whatever) - The model of an atom that has
the protons and neutrons in a
central nucleus with the
electrons in orbit about the
nucleus.
here's the nuclear model
nucleus - The center of an atom that
contains protons and neutrons.
atomic number - The number of protons in an
atom.
mass number - The sum of the number of
protons and neutrons in a
nucleus.
isotopes - Atoms of the same element
that have different numbers of
neutrons.
^^let me explain this isotope thing first!! so the atomic number is how many protons are in an atom right?? the atomic number also defines the identity of the element. so for example, most hydrogen atoms have 1 proton in their nucleus. an isotope would be a hydrogen atom that has 1 proton AND a neutron in its nucleus!
there are also 2 different types of isotopes
1) deuterium - 1 proton and 1 neutron
2) tritium (tri = 3) - 1 proton and 2 neutron
atomic symbol (this word doesn't really matter but whatever) - A one- or two-letter
representation of the name of
an element.
so right here we have the format to writing isotopes!!
x - symbol of element (atomic symbol)
z - atomic number (number of protons)
a - mass number (number of protons and neutrons combined)
below we have an example of how to write an isotope!! don't mind the change of green color btw it bothers me too but i don't have TIME to just change it like i know it takes like 10 seconds to change it and rewrite it but i could be doing other things like typing this paragraph right NOW
in this question they're asking for the symbol of an isotope of uranium!! i thought they were asking for the atomic symbol at first but theyre not soooo
the difference between an atomic symbol and a symbol is that the symbol represents the entire isotope with its mass number and atomic number while an atomic symbol only represents the element!!
take this photo as an example
ok now what is the symbol for an isotope of uranium that has an atomic number of 92 and a mass number of 235?? this is pretty easy if you couldn't tell... all you need to do is find the atomic symbol for uranium (which is U) and then put the atomic number on the bottom and the mass number on the top
like this
now to solve this, you must know the mass and atomic number first (the atomic symbol aka Fe doesn't matter if we're trying to find how many protons and neutrons there are)
the atomic number is always on the bottom, so the atomic number would be 26! that means there are 26 protons in this symbol.
now to find the neutrons, you have to subtract how many protons there are from the mass number. this is because the mass number is the neutrons and protons combined!
so if there are 26 protons, and 56 is the number of neutrons and protons combined, how many neutrons are there??
56-26=30!!! oh em gee you just learned how to find how many protons and neutrons are in an isotope symbol!!!!!!!!! 😲😲😲😲
lets do some more questions (that i think will help u understand isotopes more) !!
so here in this question its asking us which pair represents isotopes. remember, isotopes are elements that have different numbers of neutrons in their nucleus, meaning they have different mass numbers!!
lets start with example c. we have the atomic symbol of Si with the atomic number and mass number of 14 and 28. the other symbol has an atomic symbol of P with the atomic number and mass number of 15 and 31.
since isotopes are atoms of the same element that have a different number of neutrons in their nucleus, this means that this pair does NOT represent isotopes. this is because they are different elements (Si and P).
i just noticed im using periods for like the first time in forever in my blog post this is sorta weird but it feels normal to me at the moment but OH WELL as long as you guys understand atomic theory!!
now lets do example b!! for example b with have the atomic symbol of Fe with the atomic number and mass number of 26 and 56. the other symbol has an atomic symbol of Mn with an atomic number and mass number of 25 and 56.
now, when you first look at these two, you might think that they're a pair of isotopes because the atomic numbers are different. isotopes are two or more forms of the same element, meaning they have different MASS numbers, not different ATOMIC (proton) numbers.
but that thought shouldn't be crossing your mind either way because these two are made up of different elements which means they do NOT represent a pair of isotopes sooooooooo
easy way out is to just look at the element. if theyre different, theyre not isotopes, but if theyre the same look at the mass number! if the mass number is different then theyre isotopes!
remember, mass numbers are neutrons and protons combined. if the mass numbers are different for both symbols, but the atomic numbers are the same, that means there are more/less neutrons in one of the atoms!
does that make sense im not sure if that makes sense i mean technically that's just extra information to help you understand it better but if the extra information confuses you i don't know what to tell ya because it was supposed to HELP YOU but oh well i hope you understand
in the textbook it also says that its common to state the mass number after the element to indicate that its an isotope or whatever
so for example, if you have an isotope of carbon with a mass number of 12, to indicate that its an isotope you would type "carbon-12"
^^just some more extra info for you in case you're going to write an essay about isotopes idk
the key takeaways probably mean main ideas so summarize the whole lesson up!! so yeah ill be putting that at the end of every chemistry blog post im doing now
also if you want more practice to do because ur interested or something i have some exercises







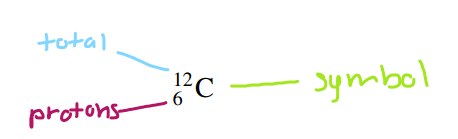



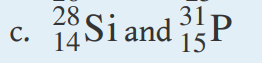





Comments
Post a Comment